ABOUT

Merlin Biomedical is a professional In-Vitro-Diagnostics (IVD) manufacturer founded in 2017. We supply range of molecular, POCT, automation HPLC system and reagents, including Infectious disease PCR tests, Rapid tests, Pharmacogenomics (PGx), Therapeutic Drug Monitor (TDM), Vitamins tests, parasite and fungus tests.

2017
Incorporation
8000 ㎡
Area
100 +
Honor
Product Services
The company integrates product research and development, production, and sales, and has a complete industrial chain in the field of diagnostic medicine
News
2023
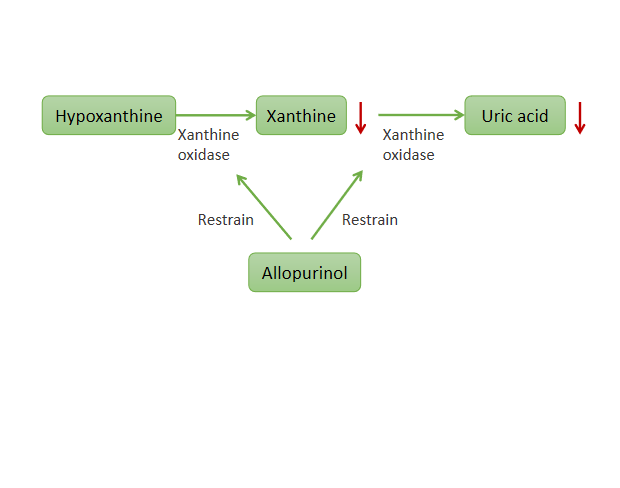
04-01Genetic testing for urico-lowering drug | is recommended before allopurinol is used to predict adver
In recent years, the incidence of high uric acid is on the rise in China. The latest data show that the scale of hyperuricemia patients in China has reached 180 million, and gout has become the second largest metabolic disease after diabetes in China, which is a health warning that cannot be ignored. Allopurinol, a drug that lowers uric acidHyperuricemia and gout are metabolic diseases caused by purine metabolism disorders. At present, the drugs commonly used in clinical uric acid reduction incl2023
03-01Genetic testing guides warfarin dosing
warfarin is an oral anticoagulant drug, which can inhibit the synthesis of vitamin K-dependent clotting factors, change the hypercoagulable state of blood, prevent thrombosis, and is suitable for the prevention and treatment of thromboembolic diseases. The stable dose of warfarin varies greatly among individualsWarfarin has a wide range of clinical indications and sufficient clinical evidence, and millions of patients worldwide are currently using warfarin.At the same time, warfarin also has man2022
04-01In addition, MerlinBiomedical COVID-19 antigen rapid test card approved CE certification!
CE2934 COVID Rapid Test card approved CE certification! MerlinBiomedical COVID-19 Antigen rapid test card obtained EU CE certification on April 5, 2022 The "CE" mark is a safety certification mark that is regarded as a passport for manufacturers to open and enter the European market.On March 11, 2022, the comprehensive team of the Joint Prevention and Control Mechanism of The State Council for the novel Coronavirus Pneumonia epidemic decided to supplement nucleic acid testing with anti2021
12-01Good news! MerlinBiomedical awarded 2021 "National High-tech Enterprise"
Recently, the Office of the Leading Group of the National high-tech Enterprise Certification Management announced the list of high-tech enterprises recognized in Fujian Province in 2021, and MerlinBiomedical was recognized as a "national high-tech enterprise". After being awarded the "specialized and special new" small and medium-sized enterprise in Xiamen City in August 2022, it added the "high enterprise" award.High-tech enterprises refer t