"The white list is finally announced!! Merrill Smith Barney New coronavirus test reagent can be expo
Date:2020-11-01
The white list of the import and Export Commerce Department of the Medical insurance Chamber of Commerce has finally been announced, and the new coronavirus detection reagent developed by MerlinBiomedical has passed the EU CE certification and can be exported
Novel Coronavirus (COVID-19) RT-PCR Kit
1. COVID-19 nucleic acid test reagent - Gold standard
The gold standard for COVID-19 diagnosis is nucleic acid detection, and the mainstream method for nucleic acid detection is fluorescent RT-PCR. Fluorescent RT-PCR is an important reference for the discharge criteria of confirmed cases.
2. Product advantages
Good specificity: generally not disturbed by other viruses.
High accuracy: Detection of two targets located on the virus ORF1ab and N genes.
Other: Wide sample applicability, set positive/negative control, reduce the risk of false positive/negative

SARS-CoV-2 Antigen Rapid Test Cassette (Colloidal Gold)
1. Antigen testing - More and more attention On August 5, 2020, The State Council held a regular policy briefing on the theme of "Improving the detection capacity of new coronavirus". The State Council said that the research and development of new coronavirus detection reagents is the key direction of emergency public relations, and it is necessary to focus on improving the speed and automation level of nucleic acid detection reagents. Simultaneous deployment of antigens, antibodies and other product development.
In view of the increasingly severe and complex global epidemic situation, the World Health Organization (WHO) and the Global Foundation for Innovative Diagnostics (FIND) are also vigorously promoting a faster and more convenient means of detection - antigen detection.
2. Antigen detection - Technical principles
The novel coronavirus gene encodes multiple structural proteins, which include multiple antigenic epitopes. Using the principle of specific binding of antigen and antibody, antibodies can detect the presence of antigen and detect whether the sample contains novel coronavirus.
3. Product advantages
Fast: Results in 15 minutes.
Convenient sampling: It can realize the detection of nasal swab, nasopharyngeal swab and other samples.
Convenient: Visual interpretation of results, no need for instruments.

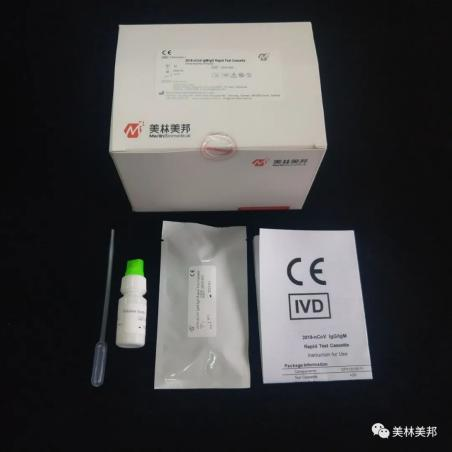
2019-nCoV IgG/IgM Rapid Test Cassette
1. "Novel Coronavirus Pneumonia Diagnosis and Treatment Protocol" (trial version 8)
According to the "Novel Coronavirus Pneumonia Diagnosis and Treatment Protocol" issued by the National Health Commission (trial version 8), the following people can be treated (patients with clinical suspicion of novel coronavirus pneumonia and nucleic acid test negative; Patients with convalescent disease and negative nucleic acid test) were tested for IgG/IgM antibodies.
2. Product advantages
Fast: Results can be read in 10 minutes.
Whole blood, serum and plasma samples can be detected.
Visual interpretation results, convenient operation, no need for instruments, a variety of scenarios can be applied.
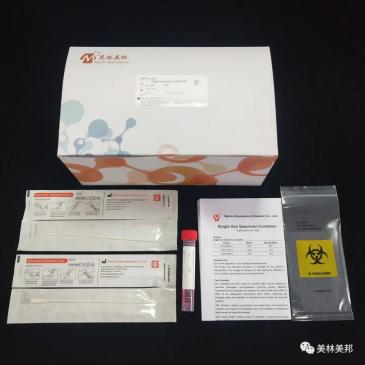
Other auxiliary testing tools (all through the EU CE certification)
In addition to the above three detection reagents that can be used for novel coronavirus, the company also provides the following auxiliary detection tools:
Disposable Sampler - (Model: N150, O150, T150)
Disposable Virus Sampling Tube - (Model: G01, G02, G03, G04)
Automatic nucleic acid Extractor - (Model: MX-48)
Nucleic acid extraction reagent - (Model: H01, H02, H03, H04)