Unstoppable, MerlinBiomedical four COVID-19 testing products passed NMPA/FDA/CE certification
Date:2020-12-01
Since the outbreak of COVID-19 and its global spread, MerlinBiomedical has stood together with the world and rapidly developed a full set of auxiliary detection tools for COVID-19 detection with its strong research and development strength - automatic nucleic acid extraction instrument, nucleic acid extraction reagent, disposable sampler, disposable virus sampling tube. These products have all obtained NMPA, FDA, CE certification, MerlinBiomedical, with products, to fight the COVID-19 pandemic at speed.
Relevant certificates show NMPA approval
FDA approval
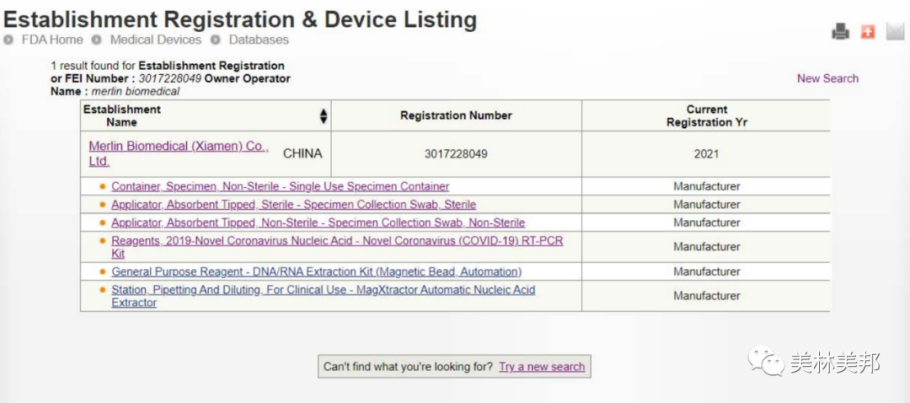
CE certification


Product features
Disposable sampler
Three models: N150 (sampling site nasopharynx), O150/T150 (sampling site oropharynx)
The product is valid for 24 months
Disposable virus sampling tube
Inactivated type (G01/G04)
Non-inactivated type (G02/G03)
Nucleic acid extraction reagent
Magnetic bead method for nucleic acid extraction
For the extraction, enrichment and purification of total nucleic acids (DNA and RNA)
Store at 0-40℃
Automatic nucleic acid extractor
High throughput, 48 samples can be processed at a time
Suitable for all kinds of samples: swabs, blood, stool, sputum, etc
Open platform, suitable for various magnetic bead method to extract reagents
Product display

① disposable sampler
Model: O150, N150
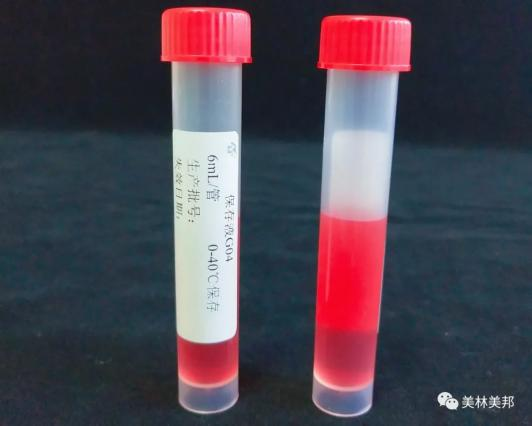
② One-time use of virus sampling tube
Model G04

③ Nucleic acid extraction reagent
Model H03

④ Automatic nucleic acid extraction instrument
Model: MX-48
MerlinBiomedical
Despite the dangers of the epidemic, we dare to stand up for the current
Use products to contribute to the global prevention and control of the novel coronavirus epidemic;
With speed, witness the company's development and mission;
With quality, for Chinese products in the world stage voice;


